Martenson geeft zijn wapenfeiten (veel 'corona-feiten' had hij al in februari en maart goed op een rijtje) en zijn fouten.
Hij pleit voor eenvoudige en goedkope middelen die de ernst van de ziekte enorm verzachten, of zelfs het ziek worden voorkomen: vitamine D, HCQ, zink, Ivermectine.
Op 19 maart publiceerde hij al over HCQ!
En nu, 6 maanden later is er alweer een studie die 43 onderzoeken over HCQ naast elkaar legt en concludeert : HCQ werkt en is niet gevaarlijk. Maar 'de officials' maken ons nog steeds wijs dat het niet werkt en gevaarlijk is ! NB: Die studie heb ik hieronder, onder de gele lijn, gepubliceerd. Of kijk HIER
Over MATH + zegt hij opmerkelijk genoeg niets, maar wie niet ernstig ziek wordt heeft al die cortisonen en ontstekingsremmers en bloed-verdunners eigenlijk ook niet nodig.
Hij pleit voor integriteit: eerlijk zijn. De Feiten laten prevaleren boven de ideologie en het eigenbelang. Citeert Warren Buffet die bij zijn beleggingen zocht naar slimme harde werkers, maar als ze niet integer waren, dan liet hij ze links liggen. (Ik vind Buffet niet zo oprecht als Martenson hem schijnt te vinden .... )
====================================
Hydroxychloroquine is Effective and Safe
for the Treatment of COVID-19,
and May be Universally Effective
When Used Early Before Hospitalization:
A Systematic Review
Source: Research Gate. (Maar op Research Gate is hij niet toegankelijk voor mij. Ik heb dus de australische Palmer Foundation gebruikt als bron.)
Conclusions Hydroxychloroquine has been shown to
have consistent clinical efficacy for COVID-19 when it is used
early in the outpatient setting, and in general would appear to work better the
earlier it is used. Overall HCQ is effective against COVID-19.
There is no credible evidence that HCQ results in worsening of COVID-19. HCQ
has been shown to be safe for the treatment of COVID-19 when responsibly used.
TY – BOOK
AU – Prodromos, Chadwick
AU – Rumschlag, Tobias
PY – 2020/09/04
SP –
T1 – Hydroxychloroquine is Effective and Safe for the Treatment of COVID-19,
and May be Universally Effective When Used Early Before Hospitalization: A
Systematic Review
DO – 10.13140/RG.2.2.29781.65765
ER –
Abstract and Figures
INTROUCTION Hydroxychloroquine (HCQ) has shown
efficacy against COVID-19 in some but not all studies. We hypothesized that
systematic review would show HCQ to be: effective against COVID-19, more
effective used earlier, not associated with worsening, and safe.
METHODS We searched PubMed, Cochrane, EmBase,
Google Scholar, and Google for all reports on hydroxychloroquine as a treatment
for COVID-19 patients. This included pre-prints and preliminary reports on
larger COVID-19 studies. We examined the studies for efficacy, time of
administration and safety.
RESULTS HCQ was found consistently effective
against COVID-19 when used early, in the outpatient setting. It was found
overall effective. No credible study found worse outcomes with HCQ use. No
mortality or other serious safety issue was found
CONCLUSIONS HCQ is consistently effective
against COVID-19 when used early in the outpatient setting, it is overall
effective against COVID-19, it has not produced worsening, it is safe.
Introduction
There is a need for effective treatment for COVID-19
infection. Hydroxychloroquine (HCQ), with or without azithromycin, has been
found to have efficacy as a treatment for COVID-19 in some studies [1, 2],
while other studies have not shown efficacy[3, 4].
Some physicians have stated that HCQ has greater efficacy if
given earlier in the course of the disease[5, 6]. Several studies showing
negative efficacy have been withdrawn due to methodological improprieties [7].
We hypothesized that HCQ clinical studies would show
significant efficacy more often than not for COVID-19; and that efficacy would
be greater if HCQ were used earlier in the course of the disease. We also
hypothesized that some studies that failed to show efficacy would be biased
against positive efficacy and that no unbiased studies would show worsening.
We also hypothesized that HCQ would be found to be safe.
Methods We searched PubMed, Cochrane, EmBase, Google Scholar, and Google for
all reports on hydroxychloroquine as a treatment for COVID-19 patients. This
included pre-prints and preliminary reports on larger COVID-19 studies. We
included papers with HCQ alone as well as in combination with Azithramycin
and/or Zinc. We excluded papers that studied Chloroquine. While Chloroquine has
shown efficacy it has a higher side effects profile than HCQ. For this reason,
and because HCQ is inexpensive and widely available we believe that future
treatment will and should focus on HCQ. It was thus our priority to examine HCQ
as fully as possible. We excluded papers that only examined hydroxychloroquine
as a means to decrease transmission of coronavirus since our focus was on
demonstrated clinical efficacy.
Reports were analyzed for efficacy, type of study, time of
intervention with HCQ during the COVID-19 disease course, and for adverse
events. Our final search was performed August 3rd, 2020.
Results
A total of 43 reports were found that examined
hydroxychloroquine treatment for COVID-19 patients. 25 found positive clinical
efficacy from using hydroxychloroquine for COVID-19 patients; 15 showed no
improvement with HCQ, and 3 showed worse clinical results in patients who
received HCQ. 11 of the studies found in our review examined HCQ efficacy on
patients in the outpatient or “day hospital” and all reported positive results
[8].
However in two of the studies [9, 10] the positive results,
while clinically important (decreased risk of hospitalization and improvement
in symptom resolution), were not statistically significant. We found 32 reports
of HCQ treatment in hospitalized patients with COVID-19. Of these 32 reports of
hospitalized patients, 14 reported good results, 15 reported no improvement and
3 reported worse results. 14 studies reported the time during treatment at
which HCQ was initiated.
In nine studies HCQ was administered within 48 hours of
admission. In six [11-16] of these nine, improvement was noted. In three it was
not [3, 17, 18]. In five studies HCQ was administered more than 48 hours after
admission or in the ICU. In two [19, 20] of these five improvement was noted.
In three it was not [21-23]. In 18 studies the time of administration was not
specified. Seven of the 43 total studies [12, 17, 20, 24-27] were chartless
retrospective studies that used only billing codes.
These studies all allowed initiation of HCQ treatment at
times that differed with initiation of the control treatment: with HCQ
presumably being chosen at the physician’s discretion in worsening patients
more in need of treatment. All such studies were felt to exhibit selection bias
against a positive result. Four additional studies [9, 10, 15, 16] had positive
trends toward efficacy that did not reach statistical significance.
In 1 study [22] 8% of the treatment group was untreated but
not excluded from the treatment group calculations. In addition the median
level of treatment was only 67% of the specified treatment. This large
undertreatment of the treatment group was also felt to bias against a positive
result. 19 of the 43 papers were pre-prints or otherwise not peer reviewed, 24
of the papers were from peer reviewed journals.
Of the eleven outpatient papers, all of which showed
positive results, 7 were peer reviewed, 4 were not. Of the 32 hospitalization
papers 17 were peer reviewed and 15 were not. Overall 12 of 24 or 50% of the
peer reviewed papers, and 11 of 19 or 58% of the non-peer reviewed papers showed
positive efficacy.
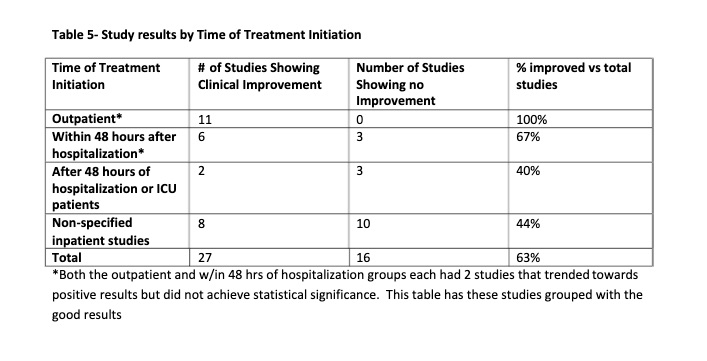
Some studies used HCQ alone, some had the addition of
azithromycin or zinc. No outcome difference was seen with the addition of
azithromycin (table 4). There were no deaths reported as a result of HCQ,
azithromycin or Zinc treatment. Increased QTc was seen but not Torsades de
Pointes. Adverse events that were felt to be likely due to HCQ treatment were
non-life threatening. All were generally self limited adverse events that
typically occur with HCQ. No permanent sequelae were described. Adverse events
are listed in Tables 1-3.
Table 1

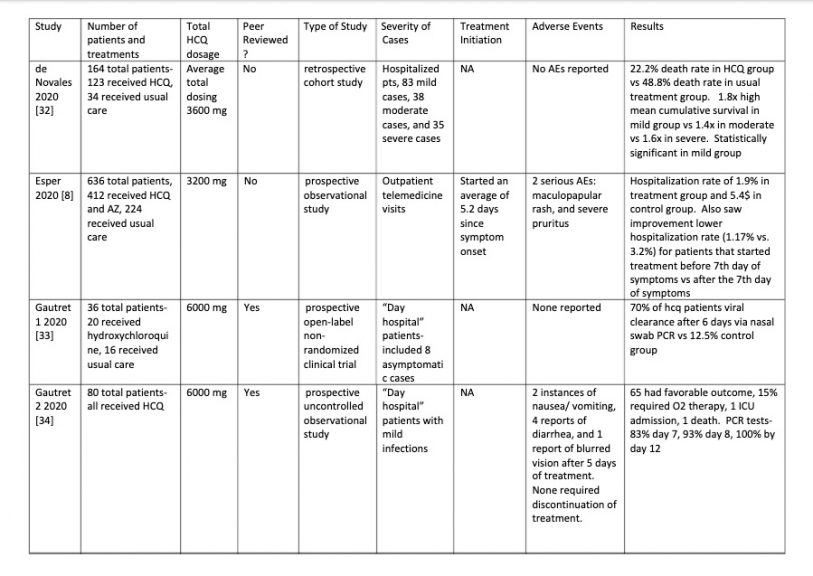



Table 2
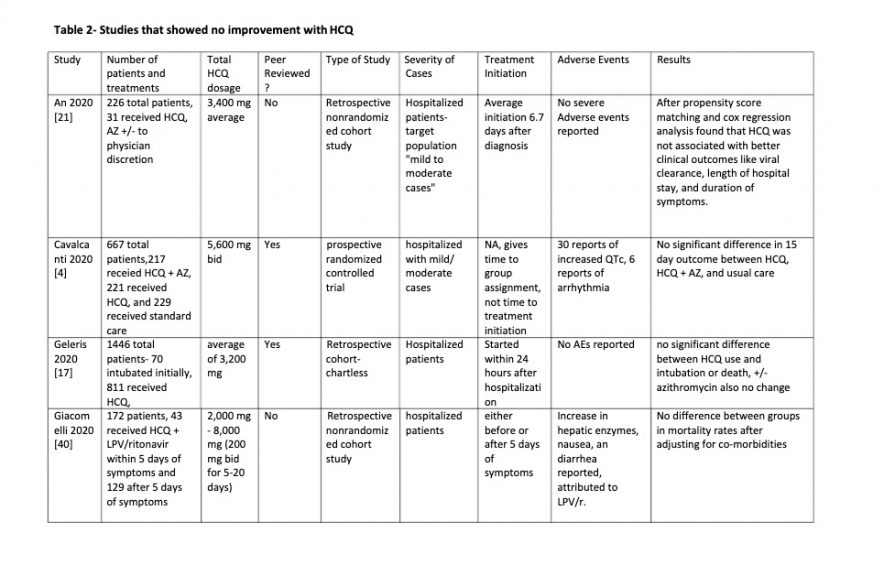
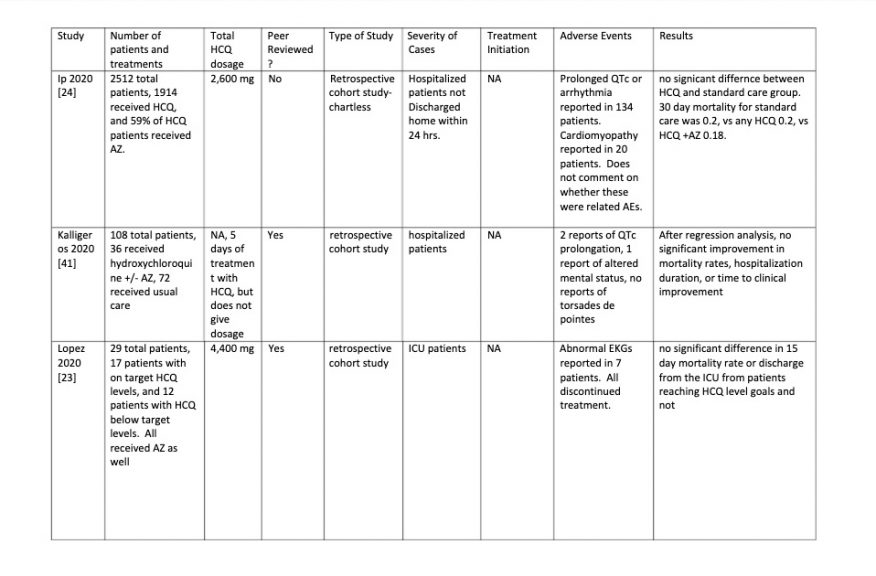



Table 3
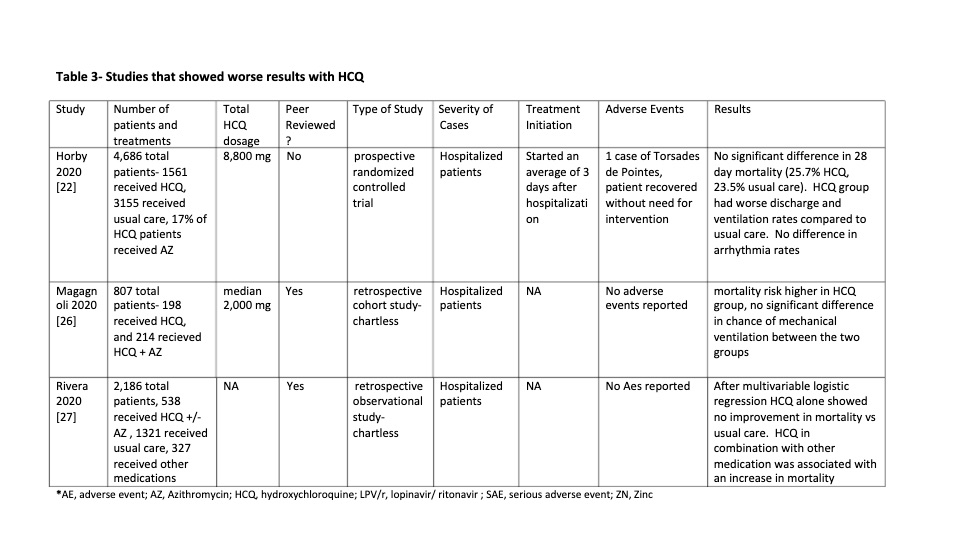
Table 4
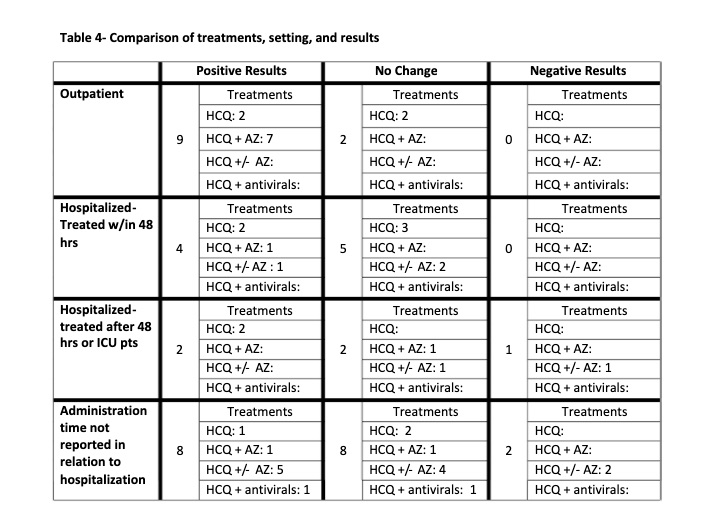
Table 5
Discussion
This study has four important findings. The first is that
HCQ appears to be consistently effective for the treatment of COVID-19 when
used early in the course of disease in the outpatient setting, and is generally
more effective the earlier it is used. The second is that overall HCQ has had
efficacy against COVID-19 in a majority of studies. The third is that there are
no unbiased studies showing a negative effect of HCQ treatment of COVID-19. The
fourth is that HCQ appears to be safe for the treatment of COVID-19 when used
responsibly.
TIMING OF HCQ USE: It was striking that 100% of
the 11 of the studies which used HCQ early in the disease on an outpatient
basis showed positive results. In two of the studies [9, 10] the benefit was
only a trend. However the effects were clinically important: in Mitja’s study
resolution of symptoms was decreased from 12 to 10 days; In Skipper’s study the
rate of hospitalization was decreased by 60%. It is likely that with higher
powering statistical significance would have been reached. In the 32 other
studies HCQ was given on an inpatient basis with more advanced disease. The
studies were divided into early, late and ICU administration times. The early
use, within 48 hours of admission showed 6 of 9 or 67% of the studies to have
positive efficacy. The two later groups, after 48 hours admission and in the
ICU showed 2 of 5 or 40% to have positive efficacy. Thus, from 100% for early
outpatient, to 67% for early hospital, to 40% for later hospital use, there
appears to be a relationship with time of initiation of treatment, and better
results the earlier HCQ is used.
OVERALL EFFICACY: 23 of the 43 studies (53%) showed a
definite positive effect of HCQ vs COVID-19. However if negatively biased
studies are removed and the clinically important positive trends from
underpowered studies are moved to the positive efficacy group the ratio changes
to 28 positive vs 9 no effect: a 75% positivity ratio of positive HCQ studies.
Interestingly none of the no-effect studies showed a clear trend toward
worsening.
RANDOMIZED CONTROLLED STUDIES (RCTs): Of the
seven RCTs two [9, 10] were in the outpatient early treated group. As described
above both studies had clinically important trends toward positive results,
although were underpowered and did not reach statistical significance. The
other five RCTs were in hospitalized patients later in disease where efficacy
seems to be less. There was 1 positive [11], 3 no-effect [4, 43, 44], and 1
negative effect [22] studies. The negative effect study, however, was biased,
as described below (“negative effect studies”), such that any negative or
no-effect result would not be valid. Thus two of two RCTs with early treatment
showed positive results, and one of three hospitalized patients had a positive
result, consistent with the general finding of better results with earlier use.
NEGATIVE EFFECT STUDIES: Three studies had data
that seemed to show worse outcomes with HCQ use. However had significant
biases. And all were in hospitalized patients when results with HCQ are less
good. Two [3, 16] of the three studies were well done studies that were
nonetheless constrained by being chartless hospitalization studies that only
used billing codes at particular time points to evaluate patients, but had no
information as to events between these time points within their hospital course
which led to initiation of treatment. Both were retrospective. Patients were
not randomized to treatment with HCQ versus other care.
Rather patients apparently received HCQ at the discretion of
the physician The time of administration of HCQ in the patients who received it
was not specified during the hospitalization. This introduces selection bias in
both studies toward treatment with HCQ for sicker patients who were faring
worse after admission who presumably would be more likely to have treatment
selected by their physician.
Attempting to normalize co-morbidities does not correct this
bias because clinical progress of COVID-19 infection is not well predicted by
pre-existing co-morbidities. This selection basis means patients who worsened
after admission who are thereby more likely to have worse outcomes would be
over represented in the HCQ treatment group.
For this reason negative results from the treatment arm of
these studies are not valid because outcomes are moved negatively. A positive
effect however would have validity since it could only occur despite the
negative selection bias, not because of it.
The third study showing worse results with HCQ was a highly
powered non-peer reviewed study whose primary outcome of 28 day mortality
actually showed no difference between the HCQ treated group and the usual
treatment group. Two of the secondary results did just barely reach
significance negatively. [22]. However the reporting of results was flawed as
follows. 8% of the treatment group patients did not receive HCQ at all; and the
median number of days of treatment for all treated patients was only 6 out of a
prescribed 9.
These facts mean that less than half of patients received
the full treatment regimen or even two thirds of the full treatment regimen,
with 1 in 12 receiving no treatment at all. These untreated and undertreated
patient outcomes were however grouped with the fully treated patient outcomes.
If HCQ has any positive effect which we believe it is well
established, this undertreatment would invalidate their borderline negative
secondary results. In addition treatment was initiated more than 48 hours after
admission when our aggregate data has shown a high incidence of no-effect
results.
The study was not blinded introducing a potential
undertreatment bias toward patients who were known by the staff to be treated
with HCQ. This study most reasonably is actually a no effects study, which is
common in already hospitalized patients such as these treated more than 48
hours after admission.
ADVERSE EVENTS: There have been fears among some
that the increased QTc seen in some patients treated with HCQ or azithromycin
would predispose to Torsades de Pointes (TDP) and then death from ventricular
fibrillation. We found no such deaths, or death from any cause related to HCQ
treatment, and indeed only 1 case of TDP at all – which resolved spontaneously
without treatment and without sequelae.
All of the adverse events which seemed attributable to HCQ
treatment in the 43 studies were typical side effects commonly seen with HCQ.
These included nausea, vomiting, diarrhea, stomach pain, headache, rash,
dizziness, itching and blurred vision. In all cases there was no indication of
persistence of symptoms after discontinuance of the HCQ.
HCQ has been used with great safety for more than 50 years,
and the relatively minor adverse events seen in these studies is consistent
with this high safety profile.
STRENGTHS AND WEAKNESSES: A strength of this
study is the large number of cohorts. A further strength is the critical
methodological study analysis heretofore not attempted to our knowledge for
these studies.
One weakness is the heterogeneity of study designs which
rendered comparison of study results challenging. Another perceived weakness of
the study could be that these include reports made outside of peer-reviewed
literature. Multiple papers reporting both improvement and no efficacy using
hydroxychloroquine that have been included in the study are either pre-prints
or preliminary results of larger trials.
Because of the unprecedented and time sensitive nature of
the SARS-COV2 pandemic the scientific community has shared data and studies on
a level unseen prior to this emergency. We believe that these reports hold
valuable information and decided to include them regardless of the way in which
they were published.
In addition we found that both the peer-reviewed and
non-peer reviewed papers showed a similar breakdown between studies showing
efficacy vs not so that bias was not introduced.
SIGNIFICANCE: We believe our findings have
substantial societal global importance since there have been numerous edicts
either preventing HCQ use for COVID-19 or limiting it to the inpatient setting
which we believe have resulted in many unnecessary deaths.
Our findings showing efficacy and safety of HCQ against
COVID-19 indicate that HCQ should be freely available to patients and
physicians who choose to use it. And it should especially be freely available
to be used on an outpatient basis before hospitalization where it appears to be
more effective and where early fears of fatal heart arrhythmias have been shown
to be unfounded[45].
This is particularly important because the only drug to show
efficacy, Remdesivir, has shown no significant benefit in a recent study
[46].It is also expensive and not widely available. Convalescent plasma has
shown benefit [47] but even this is not well validated and plasma is not
available in large numbers of doses.
Thus HCQ with proven efficacy and safety, a cost of 37 cents
per pill and thus a total treatment cost of under 20 dollars[48], versus 3,100
dollars for Remdesivir[49], as well as wide supply chain availability, would
appear to be the best COVID-19 treatment option available and needs to be
widely promoted as such.
Unfortunately the controversies surrounding HCQ have
resulted in physicians being afraid to prescribe it for reasons which have nothing
to do with medicine, and in patients being afraid to take it due to spurious
reports of danger, or fears that is not effective.
It is hoped that this study will disabuse the medical
community of these misapprehensions about efficacy and validate that it is both
efficacious and safe, and needs to be freely prescribable. Thousands of lives
may lie in the balance.
We also do not believe that randomized controlled studies
are necessary before HCQ is authorized for general use because the efficacy
seen in studies already done indicates that control patients in such studies
might die unnecessarily; and because the time delay to do any such study would
cause yet more deaths by preventing HCQ use when it is most needed – which is
immediately.
Our study has shown that good evidence of efficacy exists;
and there is no safety, cost, or supply reason to not treat now. Unnecessary
death from delayed treatment is too high a price to pay for greater certainty
of knowledge.
Many may have already died unnecessarily due to HCQ
misinformation and it is imperative that we do not further add to the toll.
Conclusions Hydroxychloroquine has been shown to
have consistent clinical efficacy for COVID-19 when it is used
early in the outpatient setting, and in general would appear to work better the
earlier it is used. Overall HCQ is effective against COVID-19.
There is no credible evidence that HCQ results in worsening
of COVID-19. HCQ has been shown to be safe for the treatment of COVID-19 when
responsibly used.
References
[1] A.d.A. Monforte, A. Tavelli, F. Bai, G. Marchetti, A.
Cozzi-Lepri, Effectiveness of Hydroxychloroquine in COVID-19 disease: A done
and dusted situation?, International Journal of Infectious Diseases, (1920).
[2] V. Guérin, P. Lévy, J.-L. Thomas, T. Lardenois, P.
Lacrosse, E. Sarrazin, N.R. de Andreis, M. Wonner, Azithromycin and
hydroxychloroquine accelerate recovery of outpatients with mild/moderate
COVID-19, Asian Journal of Medicine and Health, 18 (2020) 45-55.
[3] J. Mallat, F. Hamed, M. Balkis, M.A. Mohamed, M. Mooty,
A. Malik, A. Nusair, F. Bonilla, Hydroxychloroquine is associated with slower
viral clearance in clinical COVID-19 patients with mild to moderate disease: A
retrospective study, medRxiv, (2020).
[4] A.B. Cavalcanti, F.G. Zampieri, L.C. Azevedo, R.G. Rosa,
A. Avezum, V.C. Veiga, R.D. Lopes, L. Kawano-Dourado, L.P. Damiani, A.J.
Pereira, Hydroxychloroquine alone or in combination with azithromycin to
prevent major clinical events in hospitalised patients with coronavirus
infection (COVID-19): rationale and design of a randomised, controlled clinical
trial, medRxiv, (2020).
[5] V. Zelenko, Nunesfarma, www.nunesfarma.com.br, 2020, pp.
2.
[6] M. Million, J. Lagier, P. Gautret, P. Colson, P.
Fournier, S. Amrane, M. Hocquart, M. Mailhe, V. Esteves-Vieira, B. Doudier,
Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin:
A retrospective analysis of 1061 cases in Marseille, France. Travel Med Infect
Dis101738, 2020.
[7] J.F. Gumbrecht, Maggie, Two Coronavirus studies
retracted after questions emerge about data, CNN.com, 2020.
[8] R.B. Esper, R.S. da Silva, F. Oikawa, M. Castro, A.
Razuk-Filho, P. Junior, Empirical treatment with hydroxychloroquine and
azithromycin for suspected cases of COVID-19 followed-up by telemedicine,
Prevent Senior Institute SP, Brazil, ed. São Paulo, 25 (2020).
[9] O. Mitjà, M. Corbacho-Monné, M. Ubals, C. Tebe, J.
Peñafiel, A. Tobias, E. Ballana, A. Alemany, N. Riera-Martí, C.A. Pérez, C.
Suñer, P. Laporte, P. Admella, J. Mitjà, M. Clua, L. Bertran, M. Sarquella, S.
Gavilán, J. Ara, J.M. Argimon, J. Casabona, G. Cuatrecasas, P. Cañadas, A.
Elizalde-Torrent, R. Fabregat, M. Farré, A. Forcada, G. Flores-Mateo, E.
Muntada, N. Nadal, S. Narejos, A.N. Gil-Ortega, N. Prat, J. Puig, C. Quiñones,
J. Reyes-Ureña, F. Ramírez-Viaplana, L. Ruiz, E. Riveira-Muñoz, A. Sierra, C.
Velasco, R.M. Vivanco-Hidalgo, A. Sentís, G.B. C, B. Clotet, M. Vall-Mayans,
Hydroxychloroquine for Early Treatment of Adults with Mild Covid-19: A
Randomized-Controlled Trial, Clin Infect Dis, (2020).
[10] C.P. Skipper, K.A. Pastick, N.W. Engen, A.S.
Bangdiwala, M. Abassi, S.M. Lofgren, D.A. Williams, E.C. Okafor, M.F. Pullen,
M.R. Nicol, Hydroxychloroquine in nonhospitalized adults with early COVID-19: a
randomized trial, Annals of internal medicine, (2020).
[11] Z. Chen, J. Hu, Z. Zhang, S. Jiang, S. Han, D. Yan, R.
Zhuang, B. Hu, Z. Zhang, Efficacy of hydroxychloroquine in patients with
COVID-19: results of a randomized clinical trial, MedRxiv, (2020).
[12] S. Arshad, P. Kilgore, Z.S. Chaudhry, G. Jacobsen, D.D.
Wang, K. Huitsing, I. Brar, G.J. Alangaden, M.S. Ramesh, J.E. McKinnon,
Treatment with hydroxychloroquine, azithromycin, and combination in patients
hospitalized with COVID-19, International Journal of Infectious Diseases,
(2020).
[13] B. Davido, G. Boussaid, I. Vaugier, T. Lansaman, F.
Bouchand, C. Lawrence, J.-C. Alvarez, P. Moine, V. Perronne, F. Barbot, nImpact
of medical care including anti-infective agents use on the prognosis of
COVID-19 hospitalized patients over time, International Journal of
Antimicrobial Agents, (2020) 106129.
[14] H. Xue, Y. Liu, P. Luo, X. Liu, L. Qiu, D. Liu, J. Li,
Hydroxychloroquine treatment in COVID-19: a descriptive observational analysis
of 30 cases from a single center in Wuhan, China, Journal of Medical Virology,
(2020).
[15] O. Paccoud, F. Tubach, A. Baptiste, A. Bleibtreu, D.
Hajage, G. Monsel, G. Tebano, D. Boutolleau, E. Klement, N. Godefroy,
Compassionate use of hydroxychloroquine in clinical practice for patients with
mild to severe Covid-19 in a French university hospital, Clinical Infectious Diseases,
(2020).
[16] M. Mahevas, V.-T. Tran, M. Roumier, A. Chabrol, R.
Paule, C. Guillaud, S. Gallien, R. Lepeule, T.-A. Szwebel, X. Lescure, No
evidence of clinical efficacy of hydroxychloroquine in patients hospitalized
for COVID-19 infection with oxygen requirement: results of a study using
routinely collected data to emulate a target trial, MedRxiv, (2020).
[17] J. Geleris, Y. Sun, J. Platt, J. Zucker, M. Baldwin, G.
Hripcsak, A. Labella, D.K. Manson, C. Kubin, R.G. Barr, Observational study of
hydroxychloroquine in hospitalized patients with Covid-19, New England Journal
of Medicine, (2020).
[18] E.S. Rosenberg, E.M. Dufort, T. Udo, L.A. Wilberschied,
J. Kumar, J. Tesoriero, P. Weinberg, J. Kirkwood, A. Muse, J. DeHovitz,
Association of treatment with hydroxychloroquine or azithromycin with
in-hospital mortality in patients with COVID-19 in New York state, Jama,
(2020).
[19] B. Yu, C. Li, P. Chen, J. Li, H. Jiang, D.-W. Wang,
Beneficial effects exerted by hydroxychloroquine in treating COVID-19 patients
via protecting multiple organs, Science China Life Sciences, (2020) 1-4.
[20] B. Yu, C. Li, P. Chen, N. Zhou, L. Wang, J. Li, H.
Jiang, D.-W. Wang, Low dose of hydroxychloroquine reduces fatality of
critically ill patients with COVID-19, Science China Life Sciences, (2020) 1-7.
[21] M.H. An, M.S. Kim, B.-O. Kim, S.H. Kang, W.J. Kimn,
S.K. Park, H.-W. Park, W. Yang, J. Jang, S. Jang, Treatment Response to
Hydroxychloroquine and Antibiotics for mild to moderate COVID-19: a
retrospective cohort study from South Korea, medRxiv, (2020).
[22] P. Horby, M. Mafham, L. Linsell, J.L. Bell, N. Staplin,
J.R. Emberson, M. Wiselka, A. Ustianowski, E. Elmahi, B. Prudon, Effect of
Hydroxychloroquine in Hospitalized Patients with COVID-19: Preliminary results
from a multi-centre, randomized, controlled trial, medRxiv, (2020).
[23] A. Lopez, G. Duclos, B. Pastene, K. Bezulier, R.
Guilhaumou, C. Solas, L. Zieleskiewicz, M. Leone, Effects of Hydroxychloroquine
on Covid-19 in Intensive Care Unit Patients: Preliminary Results, International
Journal of Antimicrobial Agents, (2020) 106136.
[24] A. Ip, D.A. Berry, E. Hansen, A.H. Goy, A.L. Pecora,
B.A. Sinclaire, U. Bednarz, M. Marafelias, S.M. Berry, N.S. Berry,
Hydroxychloroquine and Tocilizumab Therapy in COVID-19 Patients-An Observational
Study, medRxiv, (2020).
[25] S. Singh, A. Khan, M. Chowdhry, A. Chatterjee, Outcomes
of Hydroxychloroquine Treatment Among Hospitalized COVID-19 Patients in the
United States-Real-World Evidence From a Federated Electronic Medical Record
Network, medRxiv, (2020).
[26] J. Magagnoli, S. Narendran, F. Pereira, T.H. Cummings,
J.W. Hardin, S.S. Sutton, J. Ambati, Outcomes of hydroxychloroquine usage in
United States veterans hospitalized with Covid-19, Med, (2020).
[27] D.R. Rivera, S. Peters, O.A. Panagiotou, D.P. Shah,
N.M. Kuderer, C.-Y. Hsu, S.M. Rubinstein, B.J. Lee, T.K. Choueiri, G. de Lima
Lopes, Utilization of COVID-19 treatments and clinical outcomes among patients
with cancer: A COVID-19 and Cancer Consortium (CCC19) cohort study, Cancer Discovery,
(2020).
[28] I. Ahmad, M. Alam, R. Saadi, S. Mahmud, E. Saadi,
Doxycycline and Hydroxychloroquine as Treatment for High-Risk COVID-19
Patients: Experience from Case Series of 54 Patients in Long-Term Care
Facilities, medRxiv, (2020).
[29] M.A. Ashraf, N. Shokouhi, E. Shirali, F. Davari-tanha,
O. Memar, A. Kamalipour, A. Azarnoush, A. Mabadi, A. Ossareh, M. Sanginabadi,
COVID-19 in Iran, a comprehensive investigation from exposure to treatment
outcomes, medRxiv, (2020).
[30] N. Bernaola, R. Mena, A. Bernaola, A. Lara, C.
Carballo, P. Larranaga, C. Bielza, Observational Study of the Efficiency of
Treatments in Patients Hospitalized with Covid-19 in Madrid, medRxiv, (2020).
[31] P. Carlucci, T. Ahuja, C.M. Petrilli, H. Rajagopalan,
S. Jones, J. Rahimian, Hydroxychloroquine and azithromycin plus zinc vs
hydroxychloroquine and azithromycin alone: outcomes in hospitalized COVID-19
patients, medRxiv, (2020).
[32] F.J.M. de Novales, G. Ramírez-Olivencia, M. Estébanez,
B. de Dios, M.D. Herrero, T. Mata, A.M. Borobia, C. Gutiérrez, M. Simón, A.
Ochoa, Early hydroxychloroquine is associated with an increase of survival in
COVID-19 patients: an observational study, (2020).
[33] P. Gautret, J.-C. Lagier, P. Parola, L. Meddeb, M.
Mailhe, B. Doudier, J. Courjon, V. Giordanengo, V.E. Vieira, H.T. Dupont,
Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an
open-label non-randomized clinical trial, International journal of
antimicrobial agents, (2020) 105949.
[34] P. Gautret, J.-C. Lagier, P. Parola, L. Meddeb, J.
Sevestre, M. Mailhe, B. Doudier, C. Aubry, S. Amrane, P. Seng, Clinical and
microbiological effect of a combination of hydroxychloroquine and azithromycin
in 80 COVID-19 patients with at least a six-day follow up: A pilot
observational study, Travel medicine and infectious disease, (2020) 101663.
[35] J.-W. Kim, E.J. Kim, H.H. Kwon, C.Y. Jung, K.C. Kim,
J.-Y. Choe, H.-L. Hong, Lopinavir-ritonavir versus hydroxychloroquine for viral
clearance and clinical improvement in patients with mild to moderate
coronavirus disease 2019, The Korean journal of internal medicine, (2020).
[36] M.S. Kim, S.-W. Jang, Y.-K. Park, B.-o. Kim, T.-H.
Hwang, S.H. Kang, W.J. Kim, H.-W. Park, W. Yang, J. Jang, Treatment Response to
Hydroxychloroquine, Lopinavir/Ritonavir, and Antibiotics for Moderate COVID 19:
A First Report on the Pharmacological Outcomes from South Korea, medRxiv,
(2020).
[37] J.-C. Lagier, M. Million, P. Gautret, P. Colson, S.
Cortaredona, A. Giraud-Gatineau, S. Honoré, J.-Y. Gaubert, P.-E. Fournier, H.
Tissot-Dupont, Outcomes of 3,737 COVID-19 patients treated with
hydroxychloroquine/azithromycin and other regimens in Marseille, France: A
retrospective analysis, Travel medicine and infectious disease, (2020) 101791.
[38] E. Sbidian, J. Josse, G. Lemaitre, I. Mayer, M.
Bernaux, A. Gramfort, N. Lapidus, N. Paris, A. Neuraz, I. Lerner,
Hydroxychloroquine with or without azithromycin and in-hospital mortality or
discharge in patients hospitalized for COVID-19 infection: a cohort study of
4,642 in-patients in France, medRxiv, (2020).
[39] M. Scholz, R. Derwand, V. Zelenko, COVID-19
outpatients–early risk-stratified treatment with zinc plus low dose
hydroxychloroquine and azithromycin: a retrospective case series study, (2020).
[40] A. Giacomelli, G. Pagani, A.L. Ridolfo, A. Oreni, F.
Conti, L. Pezzati, L. Bradanini, G. Casalini, S. Passerini, A. Torre, Early
administration of lopinavir/ritonavir plus hydroxychloroquine does not alter
the clinical course of SARS-CoV-2 infection: a retrospective cohort study,
medRxiv, (2020).
[41] M. Kalligeros, F. Shehadeh, E. Atalla, E.K. Mylona, S.
Aung, A. Pandita, J. Larkin, M. Sanchez, F. Touzard-Romo, A. Brotherton,
Hydroxychloroquine use in Hospitalized Patients with COVID-19: An observational
matched cohort study, Journal of Global Antimicrobial Resistance, (2020).
[42] J.M. Molina, C. Delaugerre, J. Le Goff, B. Mela-Lima,
D. Ponscarme, L. Goldwirt, N. de Castro, No evidence of rapid antiviral
clearance or clinical benefit with the combination of hydroxychloroquine and
azithromycin in patients with severe COVID-19 infection, Med Mal Infect, 50
(2020) 30085-30088.
[43] NIH halts clinical trial of hydroxychloroquine,
National Institutes of Health, NIH.gov, 2020.
[44] W. Tang, Z. Cao, M. Han, Z. Wang, J. Chen, W. Sun, Y.
Wu, W. Xiao, S. Liu, E. Chen, W. Chen, X. Wang, J. Yang, J. Lin, Q. Zhao, Y.
Yan, Z. Xie, D. Li, Y. Yang, L. Liu, J. Qu, G. Ning, G. Shi, Q. Xie,
Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease
2019: open label, randomised controlled trial, Bmj, 369 (2020) m1849.
[45] C.C. Prodromos, Hydroxychloroquine is protective to the
heart, not Harmful: A systematic review, New Microbes and New Infections,
(2020) 100747.
[46] C.D. Spinner, R.L. Gottlieb, G.J. Criner, J.R.A. López,
A.M. Cattelan, A.S. Viladomiu, O. Ogbuagu, P. Malhotra, K.M. Mullane, A.
Castagna, Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days
in Patients With Moderate COVID-19: A Randomized Clinical Trial, JAMA, (2020).
[47] K. Duan, B. Liu, C. Li, H. Zhang, T. Yu, J. Qu, M.
Zhou, L. Chen, S. Meng, Y. Hu, Effectiveness of convalescent plasma therapy in
severe COVID-19 patients, Proceedings of the National Academy of Sciences, 117
(2020) 9490-9496.
[48] Price Guide- Hydroxychloroquine Prices, Coupons, and
Patient Assitance Programs, Drugs.com, 2020.
[49] S. Lupkin, Remdesivir Priced At More Than $3,100 For A Course Of Treatment, National Public Radio, npr.com, 2020.
==========================================
PREVIOUSVitamin D3 mixed with hydroxychloroquine shows promise in treating COVID-19: Study
Link van plaatje boven "Research Gate" klopt niet:
ReplyDeletefile:///C:/Users/GEBRUI~1/AppData/Local/Temp/msohtmlclip1/01/clip_image002.jpg
Wolf,
Deleteik weet niet precies wat je bedoelt. Maar op 'Research Gate hebben ze volgens mij gepubliceerd. Maar daar heb ik geen toegang. Ik vond het artikel op een andere Australische site.
Ik heb het nu wat duidelijker aangegeven.
De gele hesjes hebben weer gedemonstreerd. Belarus heeft vriendelijke oom agenten vergeleken met de Franse gestapo.
ReplyDeleteEen van de grootste, meest inspirationele musici is overleden:
ReplyDeletehttps://www.theguardian.com/music/2020/sep/12/toots-hibbert-pioneering-reggae-star-dies-aged-77
Ik denk dat ik hem voor het eerst hoorde in '75; toen was hij al 10 jaar bezig. Een truly self made man die door keihard oefenen en repeteren van een tiener met een gitaar in een krottenwijk tot de Godfather van de Reggae (Veel beter dan Marley the Harley!) werd. Ook een van mijn Godfathers!
You rest in peace, my older brother, and thank you! We won't forget!!!
Ik kan er toch niet warm voor lopen, Rootman !
DeleteDan promoot ik nog maar eens John Prine en Iris Derment: https://www.youtube.com/watch?v=P8tTwXv4glY
"He's my baby and I am his honey. And I'll never let him go!"
"She swears like a sailor when she shaves her legs.."
"He's got more balls that a bigg brass monkey"
OP het andere kanaal zag ik dat E. erg negatief was over een muziekje dat door Dawg werd aangeprezen. Dan moet het haast wel goed zijn.
Prachtige muziek: https://www.youtube.com/watch?v=9uFBAjVALAU
[Dan moet het haast wel goed zijn. Prachtige muziek]
DeleteKlopt. Als bluesliefhebber zou ik zeggen: Bijna de oerblues wat die gasten spelen. Heb er van genoten.